

|
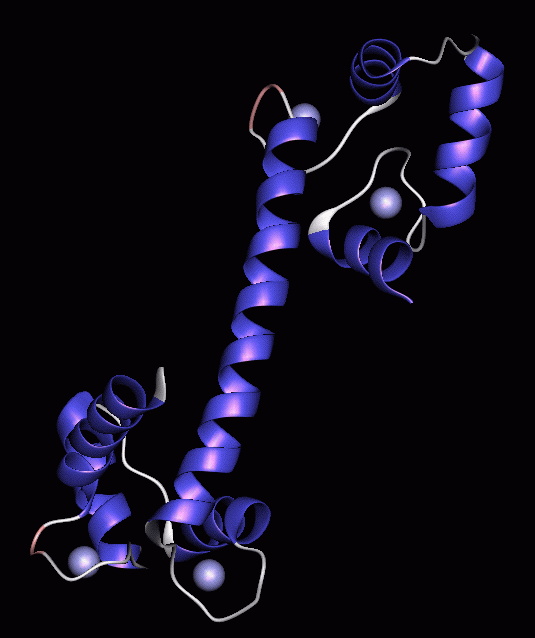
This figure illustrates the basic conformation of the calmodulin protein with secondary structure and Van der Waals illustrations of the Calcium atoms. It was this conformation that we were eager to preserve throughout the modelling process as we felt that any significant alterations to the backbone structure through the binding of Magnesium would be impossible to predict and evaluate. If this is indeed the case then the following modelling is quite realistic however if the conformation is completely altered then this modelling study is not likely to yield any valuable information. |
The next step of the modelling process is to identify the residues likely to be involved in Magnesium binding. This was done at first computationally using a program to look through the appropriate pdb file and indicate all residues which had beta carbon atoms within a given distance of the beta carbon on another acidic residue. The output of this analysis gave a useful indication of where to start looking for potential binding sites but was far from conclusive. It was then necessary to take a look at the position of these residues in real space so as to assess which were likely to pair up and coordinate a magnesium atom. This is especially important given the proviso above that any changes to the backbone structure should be minimal.
Assuming only acids to be involved in Magnesium binding, this simple analysis indicated a total of nine sites which looked to be well suited (numbers indicate residue number):
(6,7) (11,14) (47,50) (78,82) (80,83) (84,87) (119,120) (118,122) (123,127)
In addition to these acid sites we found an additional site with only one acid sidechain but an appropriate geometry for a Magnesium atom:
(29,49,45)
This seems quite possible upon deprotonation of the threonine residue in addition to another of the three suggested. Despite being slightly different in nature to the other sites, this one seems to be almost pre-organised for the Mg cation.
Having identified residues likely to be involved in Magnesium coordination it was possible to alter the orientation of these sidechains manually to be roughly positioned for the manually placed Magnesium. This was done in such a way as to move the whole of the residue sidechain and where necessary provide minor alterations to the positions of the backbone atoms of that residue without damaging the underlying framework. This produced some very peculiar looking sidechains but they could easilly be sorted out in the minimisation calculations.
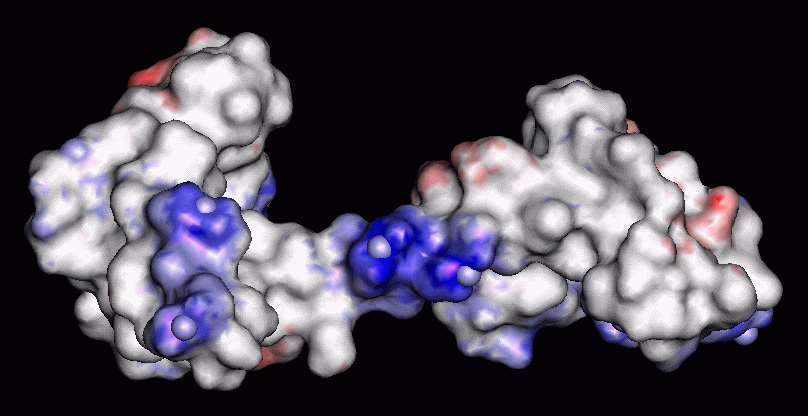
Here is a representation of the solvent accessible surface for the structure in the absence of magnesium atoms. The metal atoms were then added afterwards with a Van der Waals representation so as to indicate their space-filling. The electro-static potential colouring shows how the atoms are situated in areas of negative charge as you would expect.
Introduction Finding a Structure Sidechain Manipulation Minimisiation Conclusions